
The Hubert H. Humphrey Building, headquarters for the Department of Health and Human Services in Washington, D.C.
President Trump issued a series of Executive Orders in late July and pushed a few of them forward during his final days in office.
Unfortunately, most of the proposed rules either have few teeth, little chance of becoming binding regulation, or lack the political backing to enforce them.
For instance, the most impactful, “most favored nations” proposal would allow the Centers for Medicare & Medicaid Services (CMS) to pay the same costs for prescription drugs that other wealthy nations are charged. The pharma lobby vehemently opposes this. A similar provision in the House of Representatives’ Lower Drug Costs Now Act—allowing CMS to negotiate prices directly with drugmakers—is a nonstarter in the Senate for that same reason.
Does anyone think we’ll see Medicare beneficiaries paying the same drug prices that seniors in Europe or Canada do? Or that states will start importing lower-cost drugs en masse from those countries? Or that rebates paid by drugmakers will suddenly go away or somehow find their way to consumers?
There’s a better chance of finding oceanfront property in Kansas. However, a few much-less-publicized proposals actually have a chance for profound impact. If finalized, they are major wins for transparency and market controls on drug prices. Hopefully, the Biden Administration will carry the torch. [Hear Mike discuss on Bloomberg Radio]
Show Prices to Patients? Imagine That
Before and after the 2020 election, the Trump Administration issued two rules:
- The Hospital Price Transparency rule (CMS-1717-F2), which requires hospitals to publish payer-negotiated rates for 500 of the “most shoppable services,” and
- The Transparency in Coverage rule (CMS-9915-F), which requires private insurers to post real-time cost-sharing information, out-of-pocket coverage rates and in-network drug pricing.
What a novel idea! Allow consumers to see in advance what a procedure or prescription will cost them, and to compare prices. “For too long, American patients have been at the mercy of a shadowy system that hides crucial information,” said outgoing Health and Human Services Secretary Alex Azar. “This shadowy system needs to change.”
When initially proposed, the rule for hospitals was to take effect in 2020. They now have until January to comply. Insurers have until 2022 to post online documents for their network and non-network provider rates and in-network drug pricing. In 2023, the information will be required to be in a searchable, online shopping tool format, and in 2024, the mandate extends from 500 to all services.
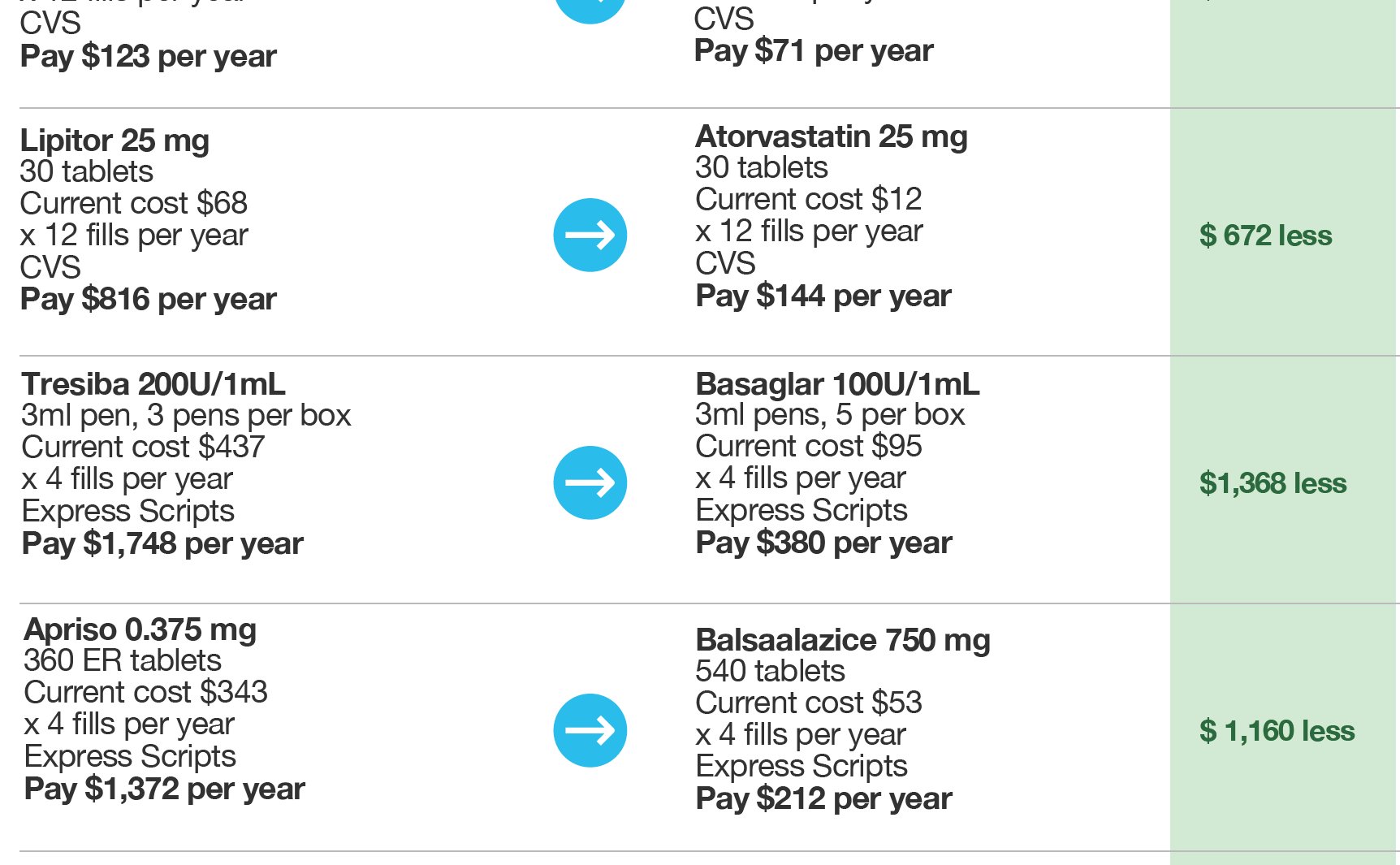 Full disclosure of costs and clinical alternatives empowers consumers to take control of their prescription costs.
Full disclosure of costs and clinical alternatives empowers consumers to take control of their prescription costs.
A Real Advantage for Medicare
Potentially great news for the 54 million Americans on Medicare: Another proposed rule (CMS-4190-P) updates the Medicare prescription drug benefit (Part D) and Medicare Advantage (Part C) plans, giving beneficiaries more choice and transparency to help reduce out-of-pocket costs.
According to CMS, the broader goal is to modernize Medicare and maximize competition among Part C and D plans. At its core is a mandate to allow members to view and compare their out-of-pocket costs for different covered drugs in advance (e.g. in a provider’s office, or when weighing plan options during open enrollment).
Part C and D plans will need to present accurate, easy-to-understand information to members in real time, in a secure patient portal or accessible by phone. The key component here goes beyond simply being able to see a drug’s cost and the member’s share of it. The rule also requires plans to display every clinically effective alternative available, thus allowing members and providers to compare all options and costs for the first time.
Compliance may get complicated and costly, but unlike the bolder, headline-making proposals, these rules for hospitals, health insurers and Medicare appear to have a realistic chance at sticking. In my eyes, they would represent the most meaningful changes in 20 years.
Consumers and payers have been clamoring for lower drug prices for more than two decades, across three administrations and 10 election cycles. When Americans can’t adhere to the medications they’re prescribed, the estimated cost to our healthcare system is $290 billion every year.
No wonder the market adoption of healthcare IT solutions built around consumer choice has been so strong. Or that GoodRx’s IPO raised $1.14 billion at a valuation of $12.67 billion. The company I founded and currently lead empowers more than 8 million members with information they need to make informed decisions on their prescription spend, whether they’re covered by an employer, commercial insurer or Medicare Advantage.
Transparency, consumerism and competition are at the heart of all these new government rules. And that’s a good thing.
Thus far, government has largely failed at policing drug costs. Its strength is establishing guiderails like those CMS is currently proposing, not negotiating on behalf of large, diverse populations of individuals. That’s best accomplished by informed consumers in a free, open market.
It’s encouraging to see Washington finally take the right approach. I urge the incoming administration to help make it reality.
For more perspective on these topics, don’t miss the Bloomberg Radio segment with our founder and CEO. (5-minute listen)
———————————————————-
STAY UP TO DATE
Sign up for our monthly newsletter, The Script, and we’ll send you a quick run-down of industry updates, thought leadership and our latest insightful blogs to keep you informed.



